Chemical reactions webquest answer key, the pivotal resource for understanding the enigmatic realm of chemical reactions, unveils the intricacies of this scientific phenomenon. As we delve into this comprehensive guide, we unravel the tapestry of chemical reactions, their diverse types, and the factors that govern their dynamics.
Embark on an enlightening journey as we decipher the mysteries of chemical transformations and unlock the secrets of this captivating scientific domain.
Chemical reactions, the cornerstone of chemistry, encompass a vast spectrum of processes that reshape the molecular landscape. From the synthesis of new compounds to the decomposition of existing ones, chemical reactions orchestrate the ceaseless dance of matter, shaping the world around us.
This webquest answer key serves as an indispensable tool for students, providing a roadmap to navigate the intricacies of chemical reactions and deepen their understanding of this fundamental scientific concept.
Chemical Reactions
Chemical reactions are processes that involve the rearrangement of atoms to form new substances. They can be represented using chemical equations, which show the reactants (initial substances) on the left and the products (final substances) on the right. For example, the reaction between hydrogen and oxygen to form water can be represented as:
H2+ O 2→ 2H 2O
Chemical reactions can be classified into different types based on the changes that occur during the reaction. The main types of chemical reactions are:
- Synthesis reactions: Two or more substances combine to form a single product.
- Decomposition reactions: A single substance breaks down into two or more products.
- Single displacement reactions: One element replaces another element in a compound.
- Double displacement reactions: Two compounds exchange ions to form two new compounds.
- Combustion reactions: A substance reacts with oxygen, releasing energy in the form of heat and light.
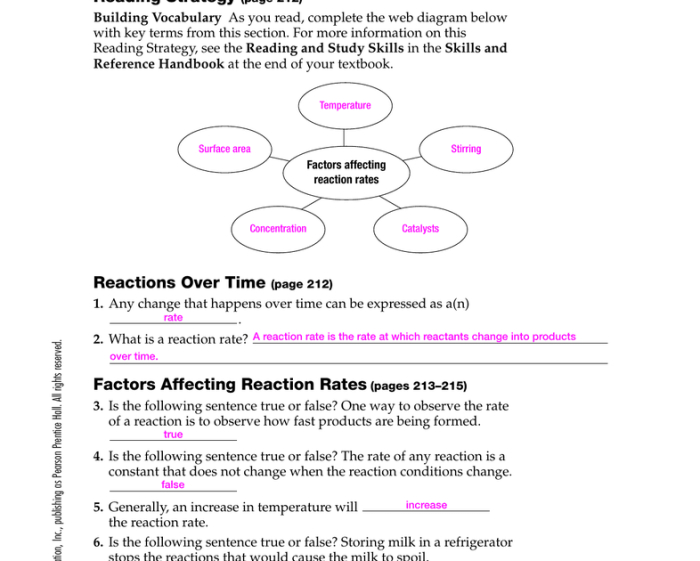
The rate of a chemical reaction is influenced by several factors, including:
- Concentration of the reactants
- Temperature
- Surface area of the reactants
- Presence of a catalyst
Webquest
A webquest is an inquiry-based learning activity that uses the internet as a primary resource. It is designed to engage students in active learning by providing them with a task to complete using information from websites and other online resources.Key
components of a webquest include:
- Introduction: Provides background information and sets the context for the task.
- Task: Clearly defines the task that students are to complete.
- Process: Artikels the steps that students should follow to complete the task.
- Resources: Provides a list of websites and other online resources that students can use to gather information.
- Evaluation: Describes the criteria that will be used to assess student work.
- Conclusion: Summarizes the learning outcomes and provides an opportunity for students to reflect on their work.
Examples of webquests that focus on chemical reactions include:
- The Chemistry of Everyday Life: Students explore the role of chemical reactions in everyday life, such as cooking, cleaning, and medicine.
- The Periodic Table of Elements: Students investigate the properties and uses of different elements, and how they react with each other.
- Chemical Reactions in the Environment: Students learn about the role of chemical reactions in environmental processes, such as photosynthesis and respiration.
Answer Key
An answer key for a webquest on chemical reactions should include questions that assess students’ understanding of the following concepts:
- The definition of a chemical reaction
- The different types of chemical reactions
- The factors that influence the rate of chemical reactions
- The applications of chemical reactions in everyday life
Questions should be clear and concise, and they should be organized in a logical order. The answer key should also provide detailed explanations for each question, so that students can understand why their answers are correct or incorrect.
Resources
| Topic | Website | Description |
|---|---|---|
| Chemical Reactions | https://www.khanacademy.org/science/chemistry/chemical-reactions | Provides interactive simulations, videos, and practice exercises on chemical reactions. |
| Periodic Table | https://www.britannica.com/science/periodic-table | Offers a comprehensive overview of the periodic table, including the properties and uses of different elements. |
| Environmental Chemistry | https://www.epa.gov/environmental-chemistry | Provides information on the role of chemical reactions in environmental processes, such as air and water pollution. |
Assessment: Chemical Reactions Webquest Answer Key
There are several methods for assessing students’ understanding of chemical reactions, including:
- Written tests: Students can be asked to answer questions about chemical reactions, including their definition, types, and factors that influence their rate.
- Laboratory experiments: Students can be asked to conduct experiments that demonstrate chemical reactions, such as the reaction between hydrogen and oxygen to form water.
- Projects: Students can be asked to create projects that explore the applications of chemical reactions in everyday life, such as a presentation on the chemistry of cooking.
Each assessment method has its own advantages and disadvantages. Written tests are a good way to assess students’ knowledge of the basic concepts of chemical reactions, but they may not be as effective at assessing their understanding of how chemical reactions work in real-world situations.
Laboratory experiments are a good way to assess students’ understanding of the practical aspects of chemical reactions, but they can be time-consuming and expensive to conduct. Projects are a good way to assess students’ creativity and ability to apply their knowledge of chemical reactions to real-world situations, but they can be difficult to grade fairly.
Extensions
Chemical reactions have a wide range of applications in various fields, including:
- Medicine: Chemical reactions are used to develop new drugs and treatments for diseases.
- Industry: Chemical reactions are used to produce a wide range of products, such as plastics, fertilizers, and fuels.
- Environmental science: Chemical reactions are used to clean up pollution and protect the environment.
Students can explore the real-world applications of chemical reactions by conducting research projects or by visiting local businesses and industries that use chemical reactions in their operations.It is important to consider the ethical implications of chemical reactions and the responsible use of chemicals.
Students can explore these issues by discussing the potential benefits and risks of chemical reactions, and by developing guidelines for the safe and responsible use of chemicals.Potential project ideas that allow students to investigate chemical reactions in depth include:
- Designing and conducting an experiment to investigate the factors that influence the rate of a chemical reaction.
- Creating a poster or presentation on the applications of chemical reactions in a particular field, such as medicine, industry, or environmental science.
- Developing a set of guidelines for the safe and responsible use of chemicals in the laboratory and at home.
FAQ Guide
What is the purpose of a chemical reactions webquest?
A chemical reactions webquest is designed to enhance students’ understanding of chemical reactions through guided online research and interactive activities.
What types of chemical reactions are covered in this webquest?
This webquest covers various types of chemical reactions, including synthesis, decomposition, single displacement, double displacement, and combustion reactions.
How can I use this answer key to assess my students’ understanding?
The answer key provides a clear and concise framework for evaluating students’ responses to the webquest questions, ensuring accurate assessment of their knowledge and comprehension.